UCLA professor’s company creates new antibacterial coating for medical devices

By Eva Danesh
Sept. 15, 2022 5:22 p.m.
This post was updated Sept. 21 at 10:55 p.m.
UCLA researchers have created a coating for implanted medical devices that will reduce their risk of causing infection.
The surface treatment was recently cleared by the FDA to be used on urinary catheters. It was created by Silq, a company co-founded by Richard Kaner, the Dr. Myung Ki Hong Endowed Chair in Materials Innovation.
Catheter-associated urinary tract infections are the most common type of hospital-associated infection – there are at least 560,000 infections and 13,000 deaths from urinary catheters alone in the U.S. every year, according to a 2016 study.
When a foreign object is introduced, the body tends to attack it in various ways, some of which attract bacteria, Kaner said, adding that if enough bacteria accumulate to eventually form a biofilm, they will become resistant to antibiotics. For example, bacteria such as E. coli will be able to double every 20 minutes once they form a biofilm, he added.
It is common practice to prescribe a course of antibiotics when patients receive a catheter for longer than a week, which is harmful as it can lead to antibiotic resistance, Kaner added.
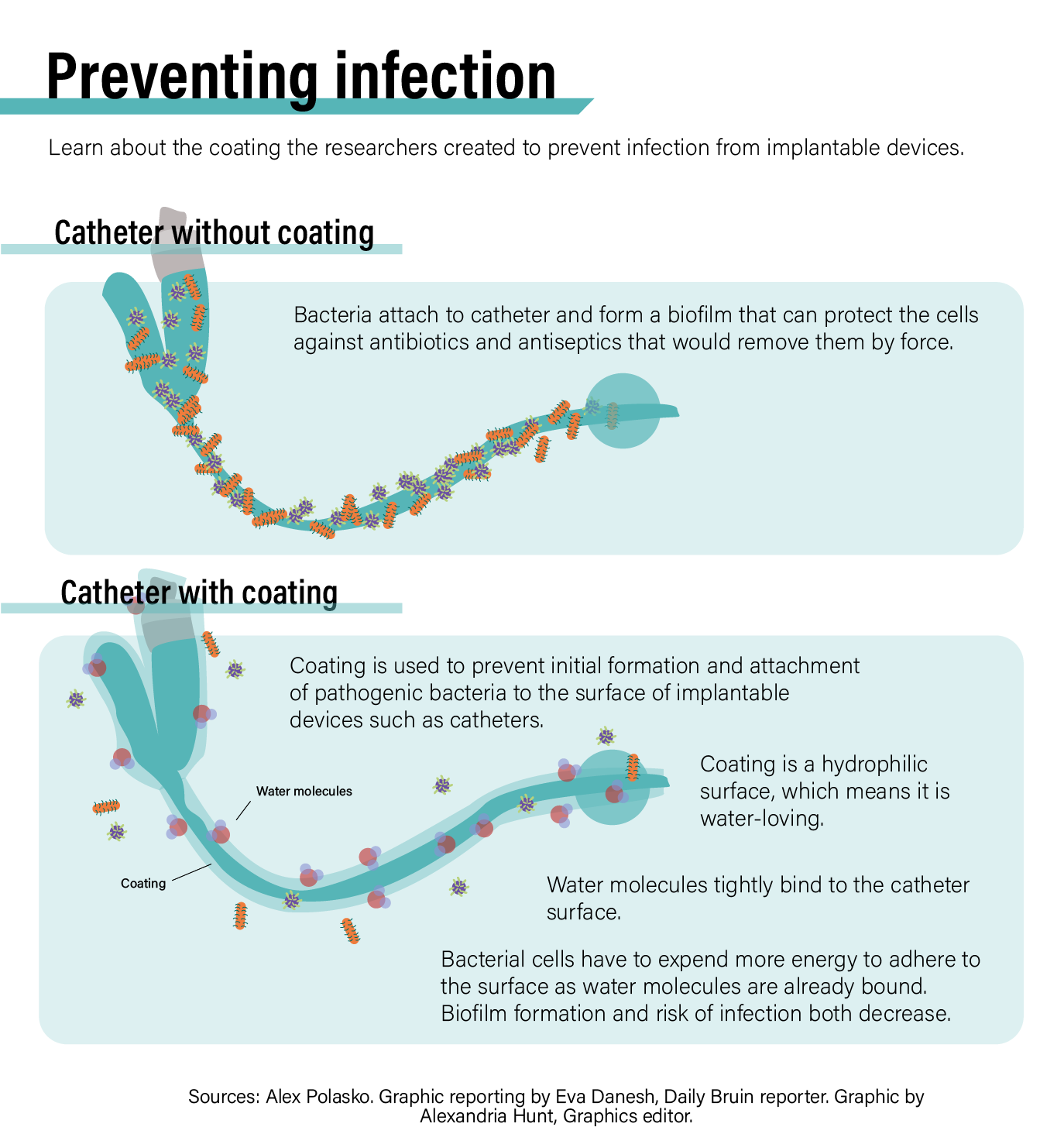
The technology for Silq’s coating – which prevents bacteria from initially attaching to surfaces of implanted devices – is rooted in decades of Kaner’s efforts in creating membranes, which did not originate in the medical field.
Following his work with gas-separating membranes, Kaner said he and his team began working on liquid-separating membranes and, after 20 years of basic science research, created a commercial product that separates oil from water and is used to clean up spills from oil fracking.
Brian McVerry, co-founder of Silq and a former doctoral student of Kaner’s, further developed the technology into a coating used in water purification to prevent filtration membranes from getting clogged because of bacteria and other materials, Kaner said.
Ethan Rao, director of research and development at Silq, said in an emailed statement that the coating’s effectiveness in purifying water served as the foundation for the technology that was developed for medical devices.
Rao, who originally joined the Kaner lab as McVerry’s undergraduate researcher, added in the statement that, since bacteria stick to water-fearing surfaces such as plastic and silicone, transforming surfaces into water-loving effectively stops infections before they begin.
“The way our coating works is by holding an ultrathin layer of water on the surface,” Rao said in the statement. “Instead of sticking to the surface and growing to infection levels, the bacteria just bounce off of our surface … and never start the growth process that leads to infections.”
Alex Polasko, a co-author alongside McVerry and Rao on the study demonstrating the coating’s effectiveness, said her lab was next to Kaner’s team as they were developing the coating and they joined the team to conduct testing on bacterial growth.
She said she tested a range of clinically relevant bacteria, including MRSA, on their initial product and consistently found bacteria did not adhere to the surface of the treated materials in comparison to untreated materials.
Polasko said the team later conducted many tests required by the FDA to ensure the material of the coating does not dissociate and that it does not pose any toxic harm to patients.
Now that urinary catheters have been shown to work with Silq’s treatment, Polasko said this technology can expand to anything going on or into the body that would benefit from being more hydrated and attracting fewer bacteria, including contact lenses and pacemakers.
“This is something that people could be using in the next couple of years – like, millions of people,” Polasko said. “My grandparents, my parents … might benefit from this technology, and that to me is just so rewarding.”
The lab’s coated catheters have undergone clinical trials, received FDA clearance, and are now being approved by various hospital systems. Los Angeles County recently approved use of these catheters, meaning that any county-affiliated hospital can now use them, Kaner said.
Urologist Dr. Evgeniy Kreydin learned of Silq’s technology from Kaner, who said Dr. Kreydin began to use it for his wheelchair-confined patients who need to use catheters continually.
One such patient had to undergo a medical procedure to replace her catheter through her abdomen every four to five days and was in regular pain, Kaner said. However, once she and her doctor agreed to switch to catheters coated in Silq’s technology, she began to have her catheter replaced and cleaned for maintenance once every several weeks and is off of all antibiotics, since there is no bacterial growth.
“This is something I never expected would happen. I’m a chemist, I’m not a physician. I never expected that to help patients directly, but we helped her,” Kaner said. “Her only request was that we do for everybody in her situation what we did for her.”
After a career of dozens of revolutionary chemical and material innovations – among others, creating the world’s hardest metal – Kaner said his work on this technology has had a different impact on him.
“This is the first time I ever did something that actually somebody can come back and say, ‘Hey, thank you, you’ve changed my life,’” Kaner said. “I’m just kind of blown away by that.”