UCLA researchers help develop improved process for synthesis gas production
(Sameera Pant/Daily Bruin)
By Zhichun Li
Jan. 22, 2020 1:51 a.m.
Researchers from UCLA and other universities have developed a stable and environmentally friendly method to produce synthesis gas.
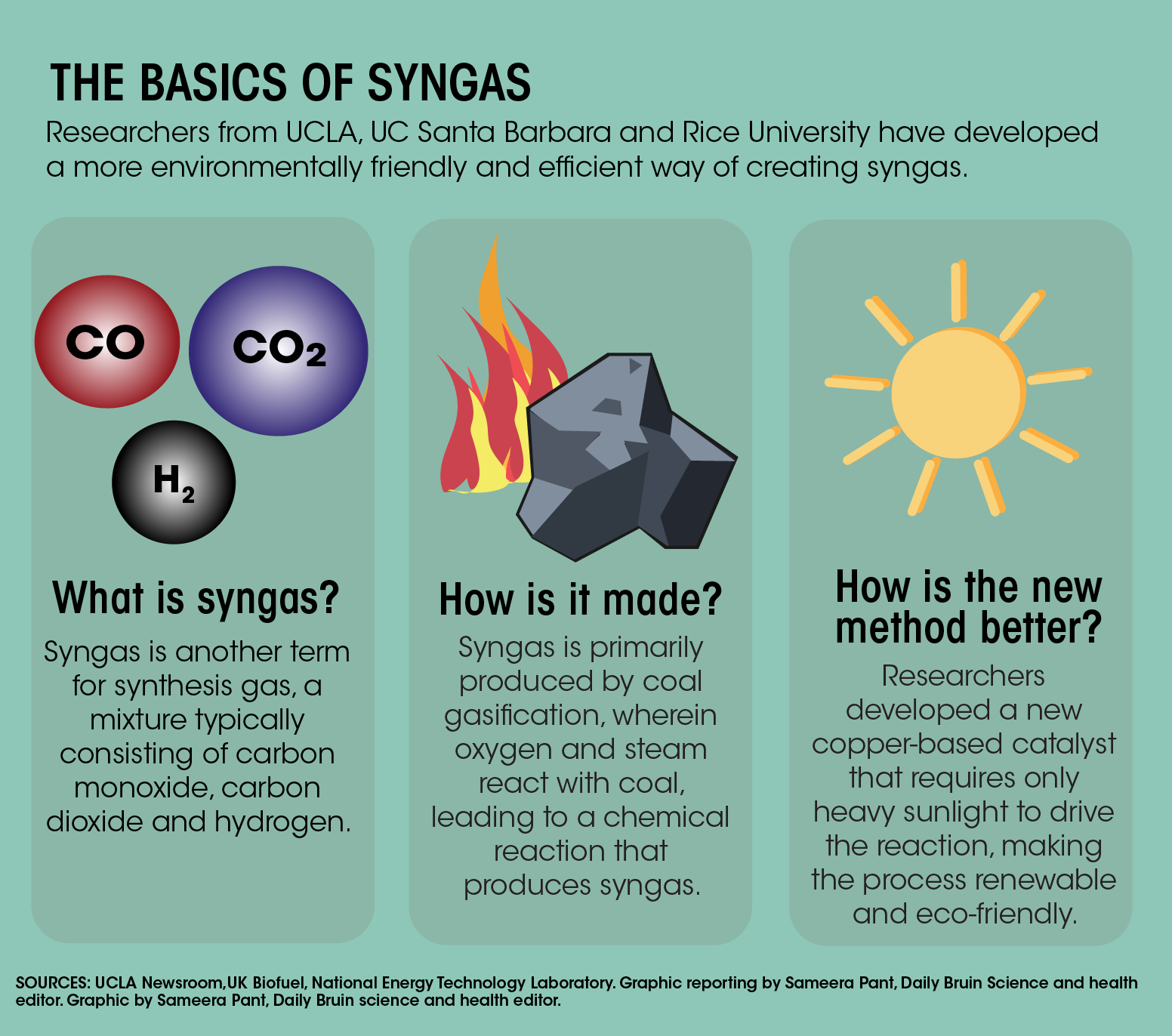
Synthesis gas, or “syngas” for short, is an industrial material made of carbon monoxide and hydrogen gas. It is conventionally used to prepare ammonia, a common ingredient in cleaning products, and methanol, a simple alcohol used in chemical industry.
Coal-derived syngas provides an alternative way to produce gasoline for countries and regions without access to oil, said Emily Carter, corresponding author of the paper and executive vice chancellor and provost of UCLA.
“We are taking two very potent greenhouse gases and making them into syngas,” Carter said.
The researchers from UCLA, as well as UC Santa Barbara and Rice University, improved on the dry reforming process, a method of producing syngas, making it stable and more environmentally friendly. The new method was detailed in a study, published in Nature Energy on Jan. 6.
Currently, the most prevalent method for syngas production is steam methane reforming, said Linan Zhou, the study’s first author and a postdoctoral researcher at Rice University. Steam reforming takes place when hydrocarbons and water vapor react at a very high temperature of up to 1,000 degrees Celsius.
This high temperature is usually achieved by burning traditional fossil fuels, which release large quantities of carbon dioxide into the atmosphere, Carter said. An alternative method, dry reforming of methane, especially if it can be done at low temperatures, provides a much greener way to produce syngas, she added.
The dry reforming process consumes carbon dioxide and methane. Carbon dioxide can be extracted from industrial processes such as steel and cement manufacturing and from power plants using fossil fuels, Carter said.
Methane can be captured from landfills, abandoned natural gas wells and as side products from human activities such as raising cattle, she said.
However, current dry reforming suffers from “coking,” when carbon compounds build up and encase the reaction’s metal catalyst rapidly, preventing the process from continuing, Zhou said.
Zhou said his group studied the effects of adding the element ruthenium to a copper-based catalyst. The team then developed the ruthenium-doped catalyst into a material with special surface properties to resolve the issue of coking.
According to the study, the improved process is stable and can last about 50 hours. Moreover, researchers found that by shining light onto the new catalyst’s surface, it was able to reduce the temperature required to start the reaction significantly.
The light also energized hydrogen atoms and prevented them from reacting with oxygen atoms to form water. This also prevented coking as carbon atoms reacted with oxygen instead of with each other.
John Mark Martirez, an assistant project scientist at UCLA and second author of the paper, analyzed the reaction with computational tools that simulated the distribution of electrons in molecules and other chemical systems.
Martirez said the simulations found that the presence of ruthenium could greatly enhance the carbon-hydrogen bond-breaking process, which is the initial step for syngas production.
Since the ruthenium particles are thinly spread throughout the surface of the catalyst, the carbon atoms made by the bond-breaking process cannot form carbon-rich compounds to cover the surface, Martirez said.
Ruthenium binds carbon atoms stronger than copper. As a result, the carbon atoms remained concentrated at the ruthenium sites, distant from each other, and had sufficient time to form carbon monoxide, Martirez said.
Analysis showed that the copper particles can efficiently absorb energy from the light and direct the collected energy input toward the ruthenium site to facilitate bond breaking.
The copper-ruthenium compound could also be a good catalyst for other chemical processes, such as ammonia synthesis, Zhou said.

