Researcher investigates potential treatment for paralysis, draws mixed reactions
(Courtesy of Mayo Clinic News Network)
By Teddy Rosenbluth
Oct. 15, 2018 1:16 a.m.
This post was updated Oct. 19 at 5:08 p.m.
Reggie Edgerton never intended to make a man with paralysis walk. In fact, the first time one of his patients moved his legs in 2013, the professor of neurosurgery thought there must have been a mistake.
“We thought, ‘This is crazy,’” Edgerton said. “Maybe the person really wasn’t completely paralyzed in some way.”
But in case after case, Edgerton and his colleagues watched their patients, one of whom had had paralysis for 20 years, regain control of paralyzed body parts.
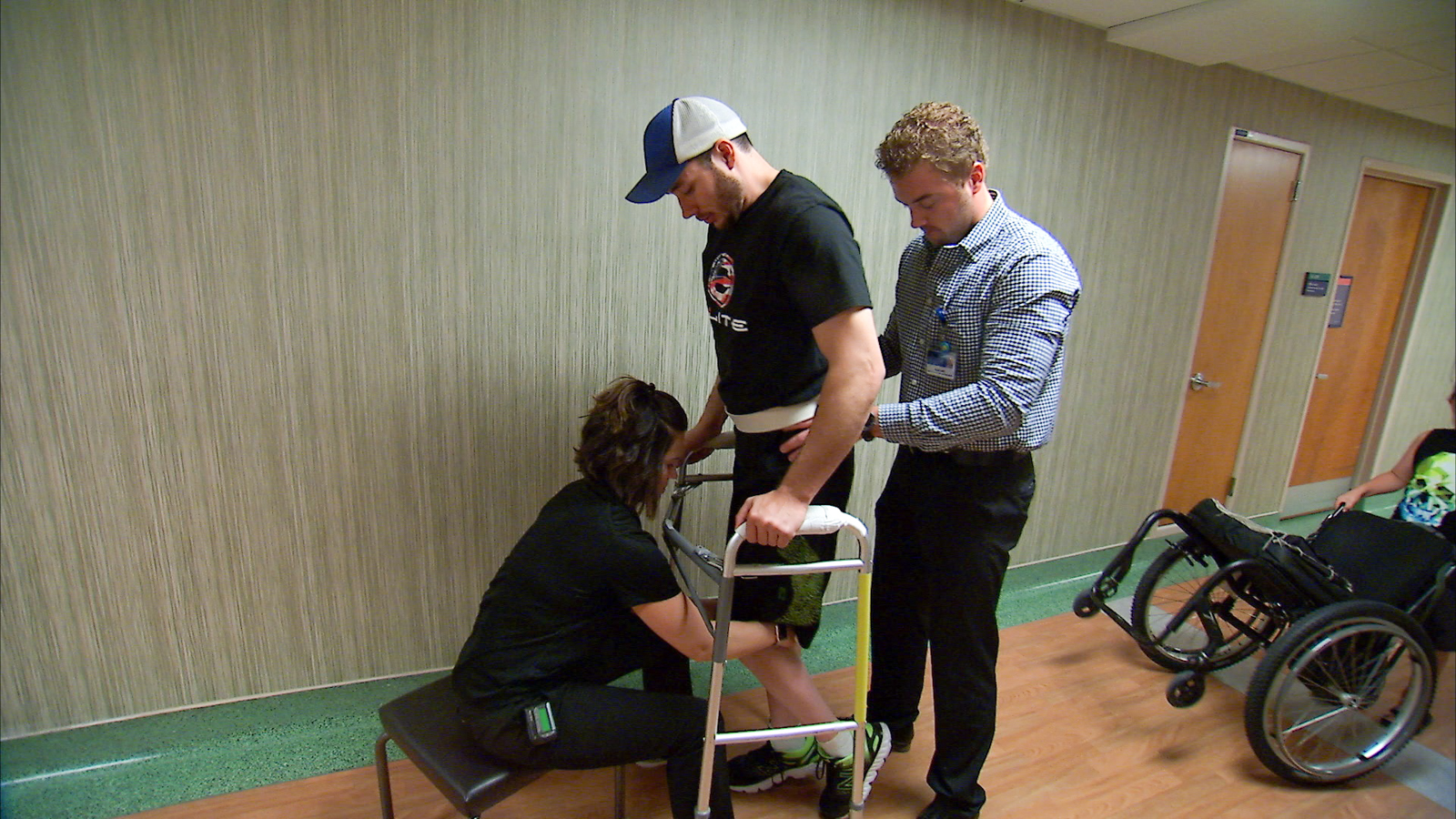
Edgerton published his most recent case study in Nature Medicine, discussing a 29-year-old man who was able to walk the length of a football field years after being paralyzed in a snowmobile accident.
This is the first time, as far as Edgerton is aware, that a patient with complete loss of function in their muscles has been able to walk by their own volition.
Edgerton’s results call a commonly held belief about the nature of paralysis into question.
Most doctors believe neural spine cells, which relay information from the brain to the rest of the body, are killed in the blunt force of a paralyzing accident, creating a dead zone that neural messages cannot cross.
In Edgerton’s experiments, small chips with 16 electrodes delivering mild electrical impulses were implanted between the spinal cord and bone. Somehow, the brain’s messages were able to cross the gap.
“Everybody, including us, thought no information could cross the lesion,” Edgerton said. “We still don’t know how it happened.”
The success of this technology demonstrates in cases of paralysis, neural cells aren’t necessarily dead, as many doctors believe – they just cannot transmit messages on their own.
Researchers think that when prompted with electrical stimulation, damaged neurons are more readily able to fire. That’s why, when the device is on, a patient with paralysis is able to send the command “walk” from the brain, through the lesion, and to the legs.

Kristin D. Zhao, a researcher at Mayo Clinic and author on the paper, said it is important to remember that this is not a treatment for paralysis, as patients still spend most of their days in a wheelchair.
The technology still has the potential to improve quality of life for individuals with paralysis, Edgerton said. As the patients started walking, Edgerton said, many bodily functions that had become inactive since paralyzation, including bladder control and sexual function, started to show improvements.
The treatment also improved some of Edgerton’s patients’ abilities to regulate their own body temperatures, which, when left untreated, can cause hypothermia. A couple of patients even reported regaining feeling in their legs with the device.
Edgerton said his research has not been met with open arms from the medical community. Doctors told him that it was impossible to restore function after a year of paralysis and suggested his patients weren’t completely paralyzed to begin with.
“That’s generally the way science is – if it’s something really different, it takes a while to change the opinion (of the medical community),” Edgerton said.
Daniel C. Lu, a neurosurgeon at Ronald Reagan UCLA Medical Center, said while he is hopeful about the experiments, he can understand why some doctors might be skeptical. He added the study published last week only evaluated one participant and cannot necessarily be generalized to other patients with paralysis.
He also said the improvement in quality of life is not significant enough to justify the steep price of the procedure, about $130,000.
“If you’re going to spend this much money on an intervention, you have to demonstrate changes in daily living, which this intervention hasn’t been demonstrating,” Lu said.
He said even with this treatment, patients would still have to rely on caretakers in their daily lives. Still, Lu said he thinks most major interventions start off with these types of problems, so he expects many of these problems will be addressed as it develops.
Edgerton says the hesitancy from the medical community has made it difficult for his project to get funding. He said many individuals with paralysis or their parents have emailed him, asking for help, but limited funds have made it difficult to incorporate the treatment into mainstream medicine.
“It’s frustrating because we know in most of those cases we can help them but the problem is we don’t have FDA approval yet,” he said. “We could have already had it to the market if we had the resources.”
Edgerton said he thinks his study shows that treatment for paralysis does not have to be limited to palliative care.
“After a year … no one really pays much attention to (patients with paralysis),” he said. “(Doctors) just keep them from dying.”
Edgerton said his new intervention could change the way doctors and patients view a paralysis diagnosis. To him, there is no question that paralysis is no longer a permanent condition.